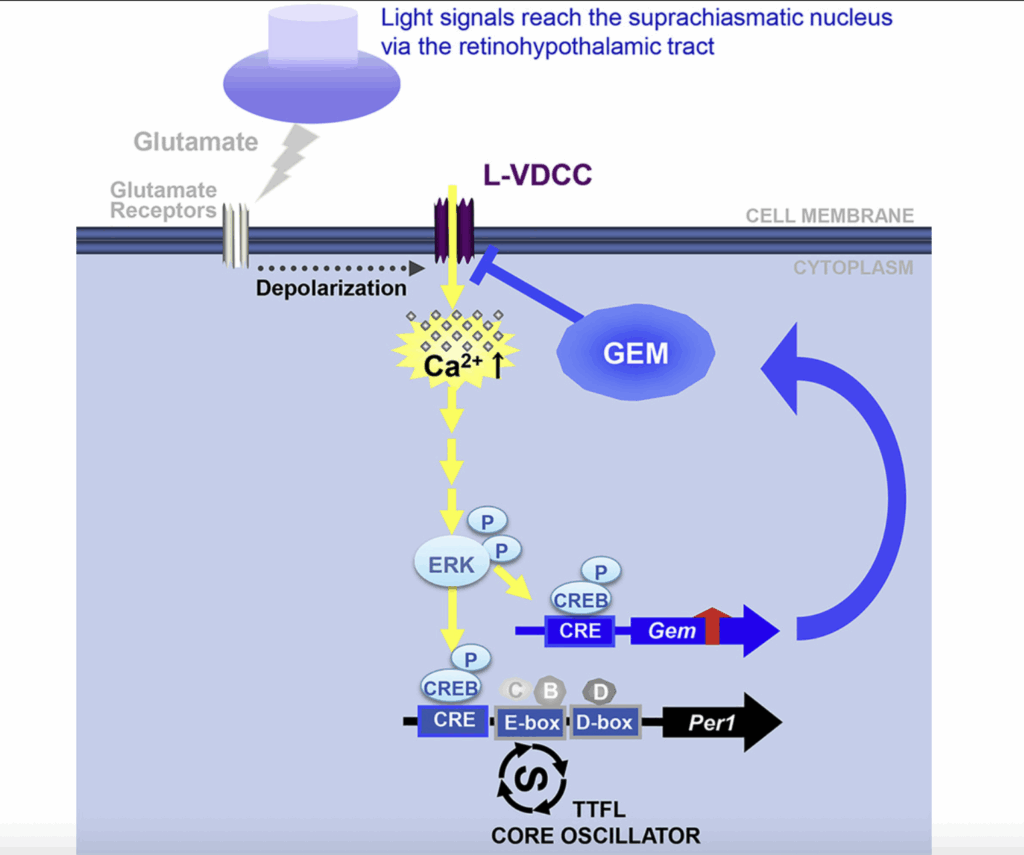
A light-induced small G-protein gem limits the circadian clock phase-shift magnitude by inhibiting voltage-dependent calcium channels
Author links open overlay panelMasahiro Matsuo 12, Kazuyuki Seo 1, Akiyuki Taruno 34, Yasutaka Mizoro 1, Yoshiaki Yamaguchi 1, Masao Doi 1, Rhyuta Nakao 5, Hiroshi Kori 6, Takaya Abe 4 7, Harunori Ohmori 4, Keiko Tominaga 89, Hitoshi Okamura 1101
Calcium signaling is pivotal to the circadian clockwork in the suprachiasmatic nucleus(SCN), particularly in rhythm entrainment to environmental light-dark cycles. Here, we show that a small G-protein Gem, an endogenous inhibitor of high-voltage-activated voltage-dependent calcium channels (VDCCs), is rapidly induced by light in SCN neurons via the calcium (Ca2+)-mediated CREB/CRE transcriptional pathway.
Neuronal calcium signaling: function and dysfunction
Marisa Brini 1, Tito Calì, Denis Ottolini, Ernesto Carafoli
Calcium (Ca(2+)) is a universal second messenger that regulates the most important activities of all eukaryotic cells. It is of critical importance to neurons as it participates in the transmission of the depolarizing signal and contributes to synaptic activity. Neurons have thus developed extensive and intricate Ca(2+) signaling pathways to couple the Ca(2+) signal to their biochemical machinery…[]…The impaired ability of neurons to maintain an adequate energy level may impact Ca(2+) signaling: this occurs during aging and in neurodegenerative disease processes. The focus of this review is on neuronal Ca(2+) signaling and its involvement in synaptic signaling processes, neuronal energy metabolism, and neurotransmission. The contribution of altered Ca(2+) signaling in the most important neurological disorders will then be considered.
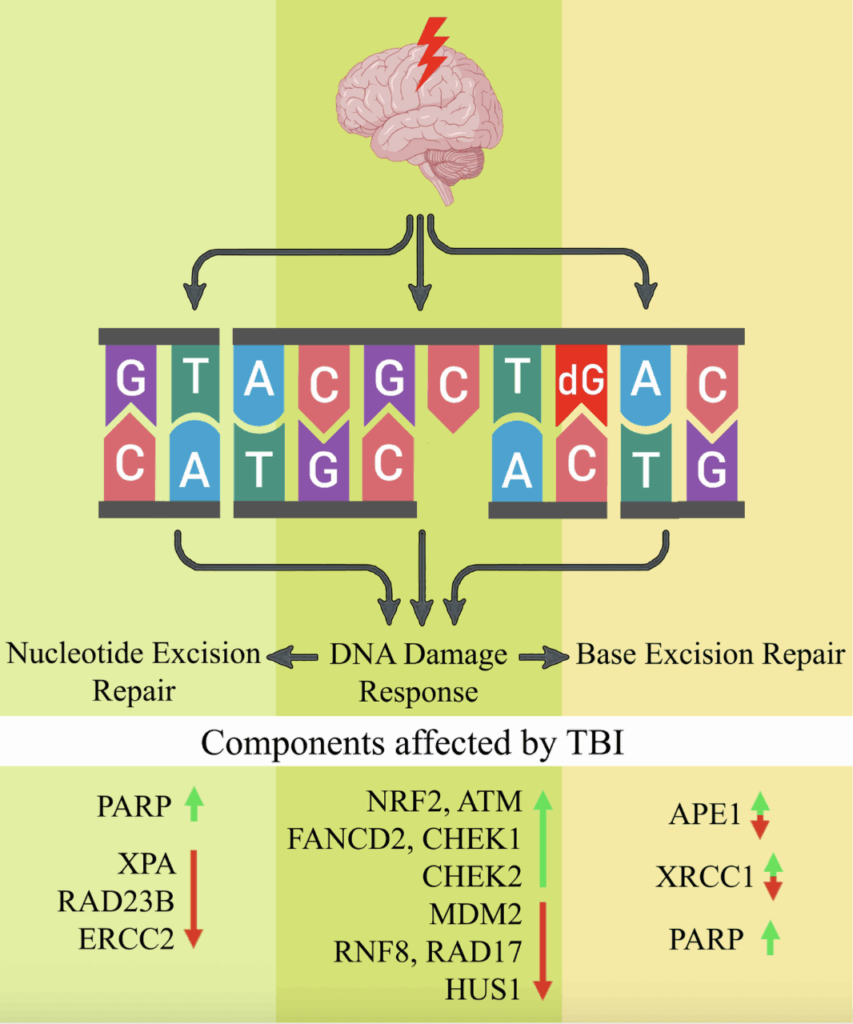
DNA damage and repair following traumatic brain injury
Charles K. Davis a, Raghu Vemuganti
Although several DNA repair pathways are induced following TBI, the simultaneous downregulation of some of the genes and proteins of these pathways leads to an aberrant overall DNA repair process. In many instances, DNA damages escape even the most robust repair mechanisms, especially when the repair process becomes overwhelmed or becomes inefficient by severe or repeated injuries. The persisting DNA damage and/or lack of DNA repair contributes to long-term functional deficits.
https://www.sciencedirect.com/science/article/pii/S0969996120304186